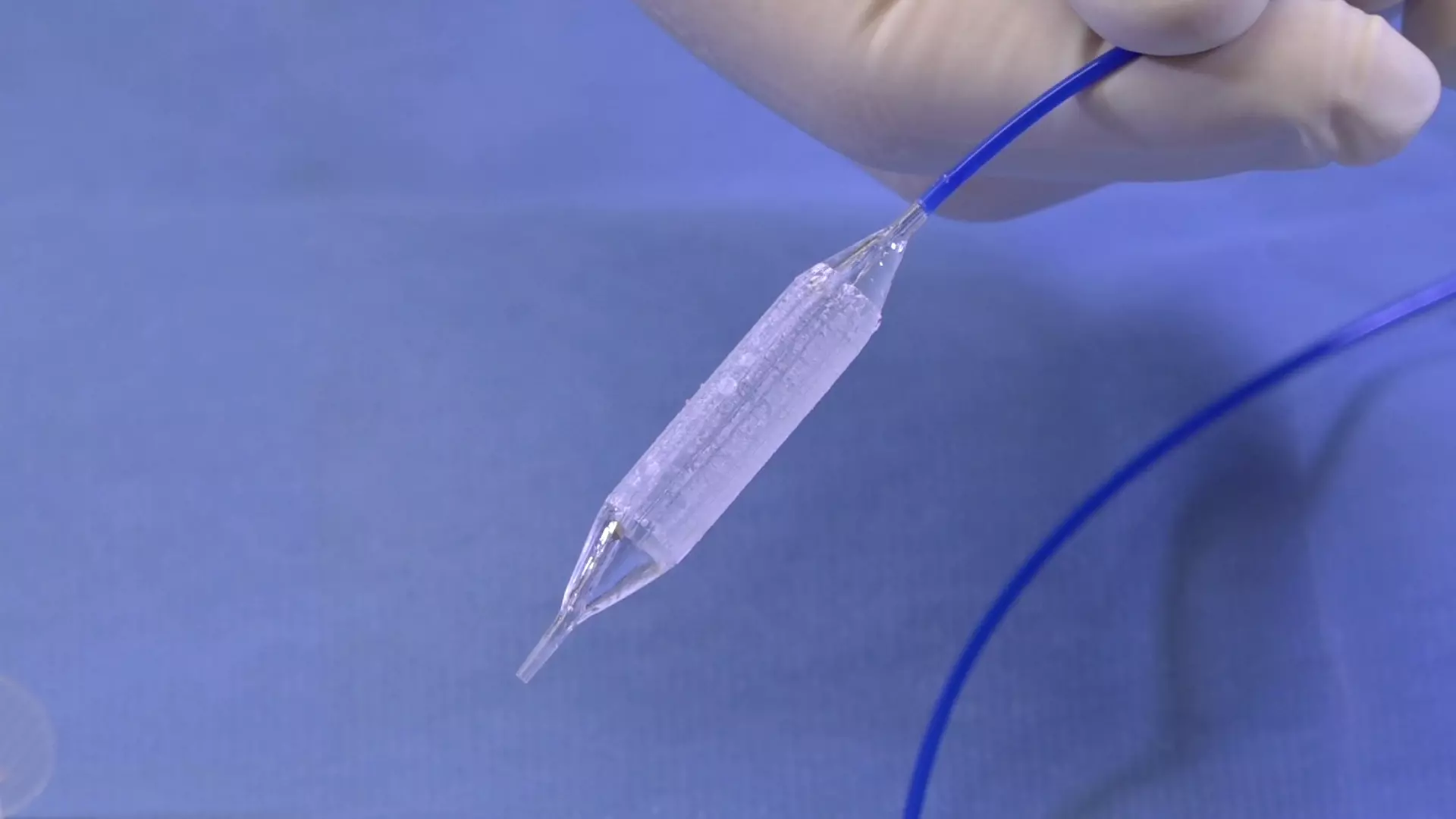
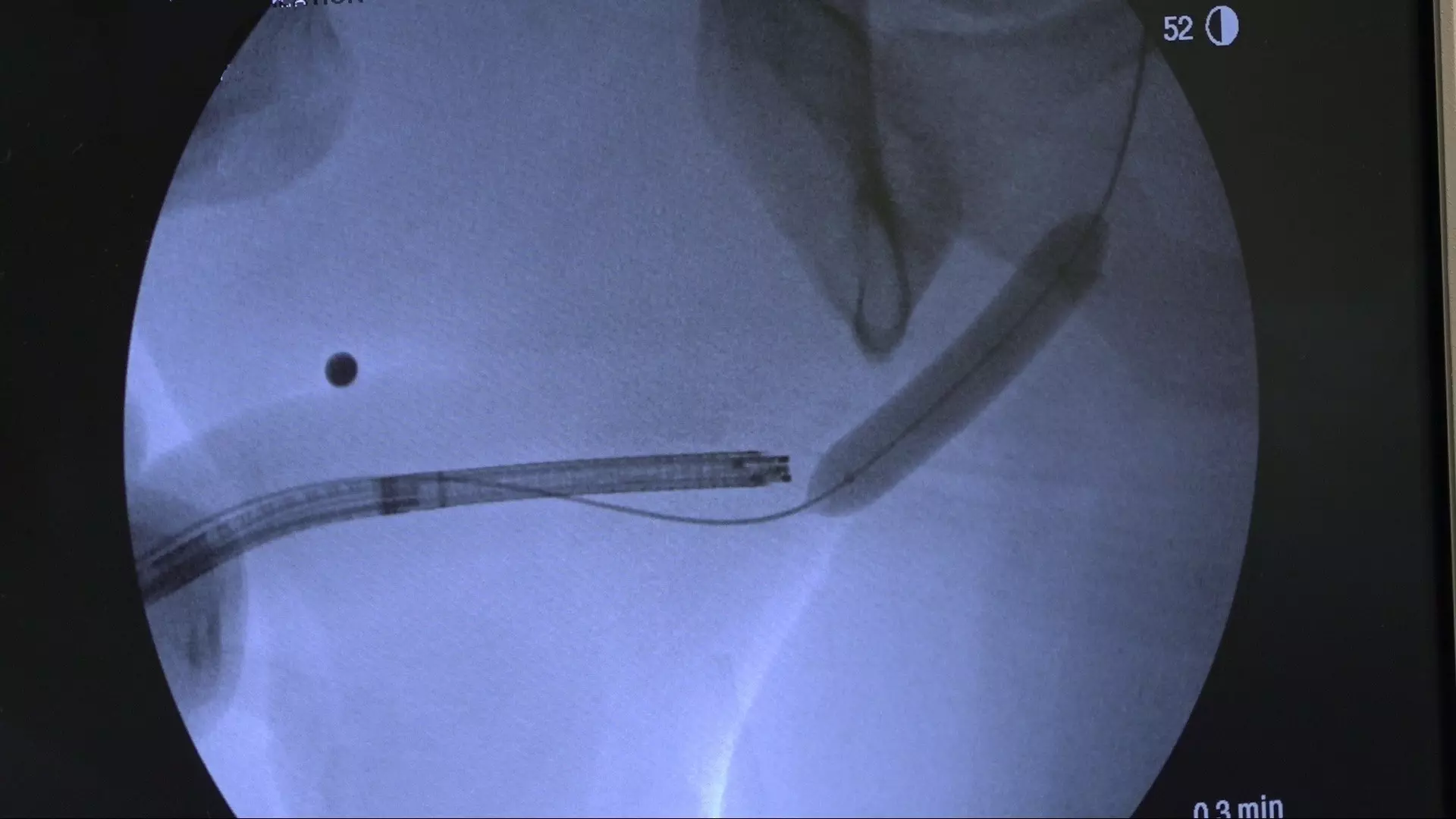
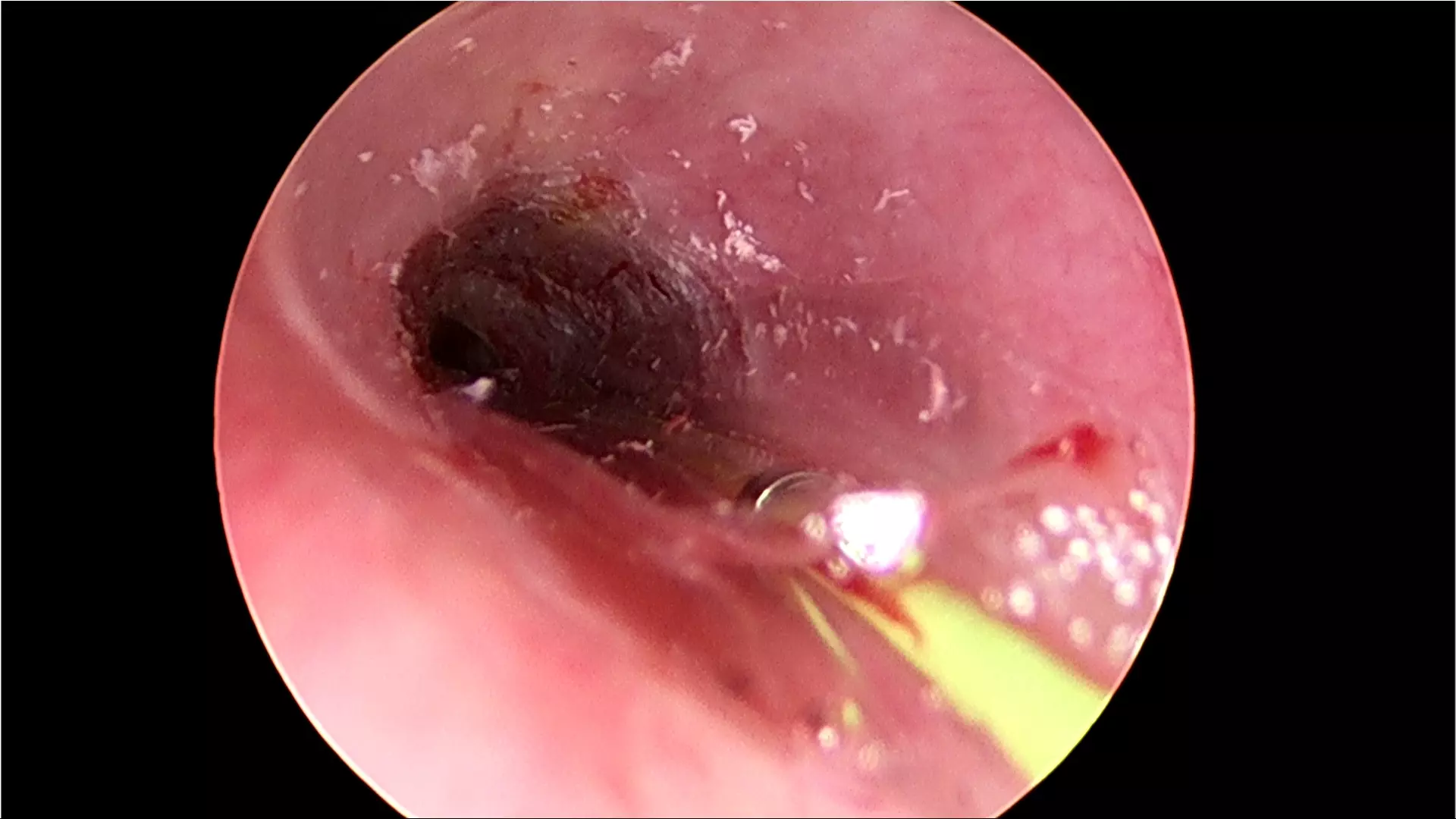
How durable are the outcomes?
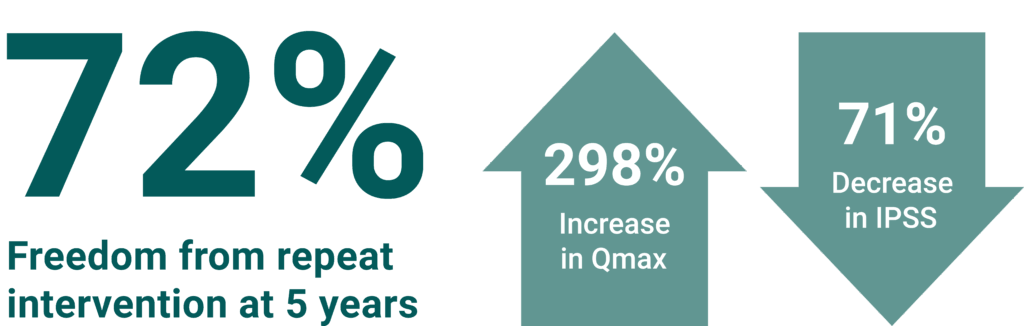
At 5 years, durability continued with 72% FFRI* 1, with a 298% increase in Qmax* (Baseline 5mL, 5-year 19.9mL)¹ and a 71 decrease in IPSS* (Baseline 22.5, 5-year 7.2).¹ Qmax & IPSS are using FCF* rates – Includes worst observed value carried forward for subjects undergoing repeat intervention of the study stricture (i.e. clinical failures).
*Qmax = Maximum Urinary Flow Rate
*FFRI = Freedom From Repeat Intervention (inc. self-catheterization)
*IPSS = International Prostate Symptom Score
*FCF = Failure Carried Forward
Simple | Easily achieve relief
Safe | Proven on a difficult patient population1,2
Durable | The breakthrough treatment to break the stricture cycle
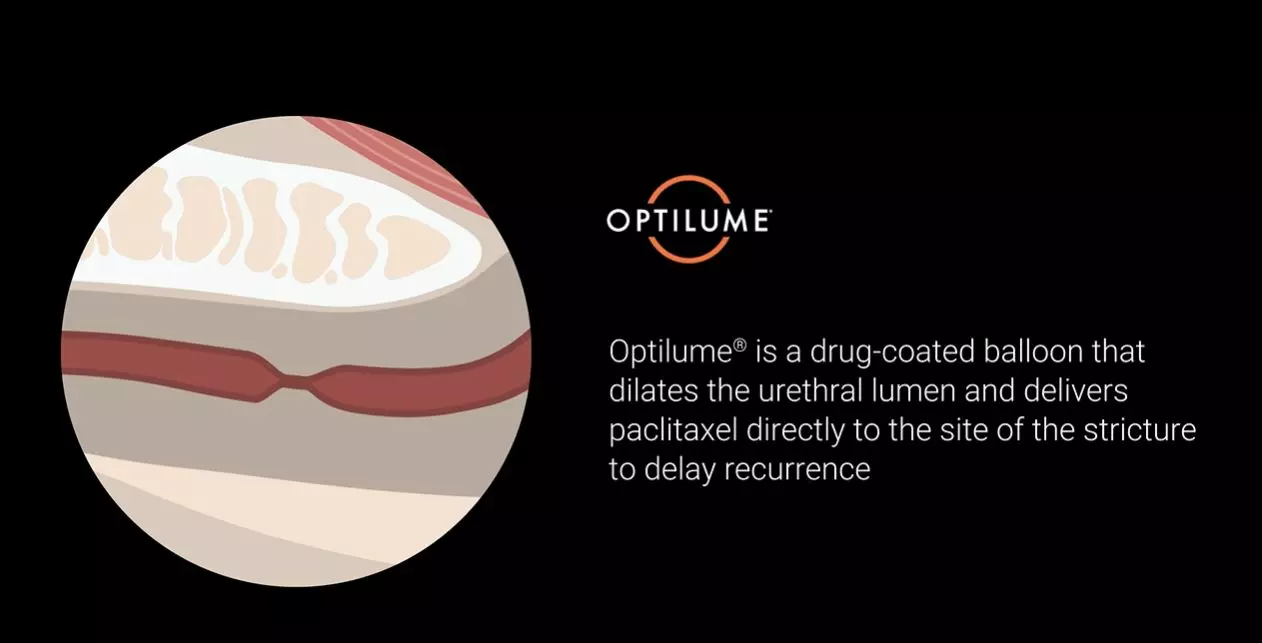
For patients who have failed prior endoscopic treatment, today there is Optilume® — a breakthrough urethral drug-coated balloon treatment that dilates the urethral lumen and delivers paclitaxel directly to the stricture, significantly reducing the incidence of stricture recurrence.1,2