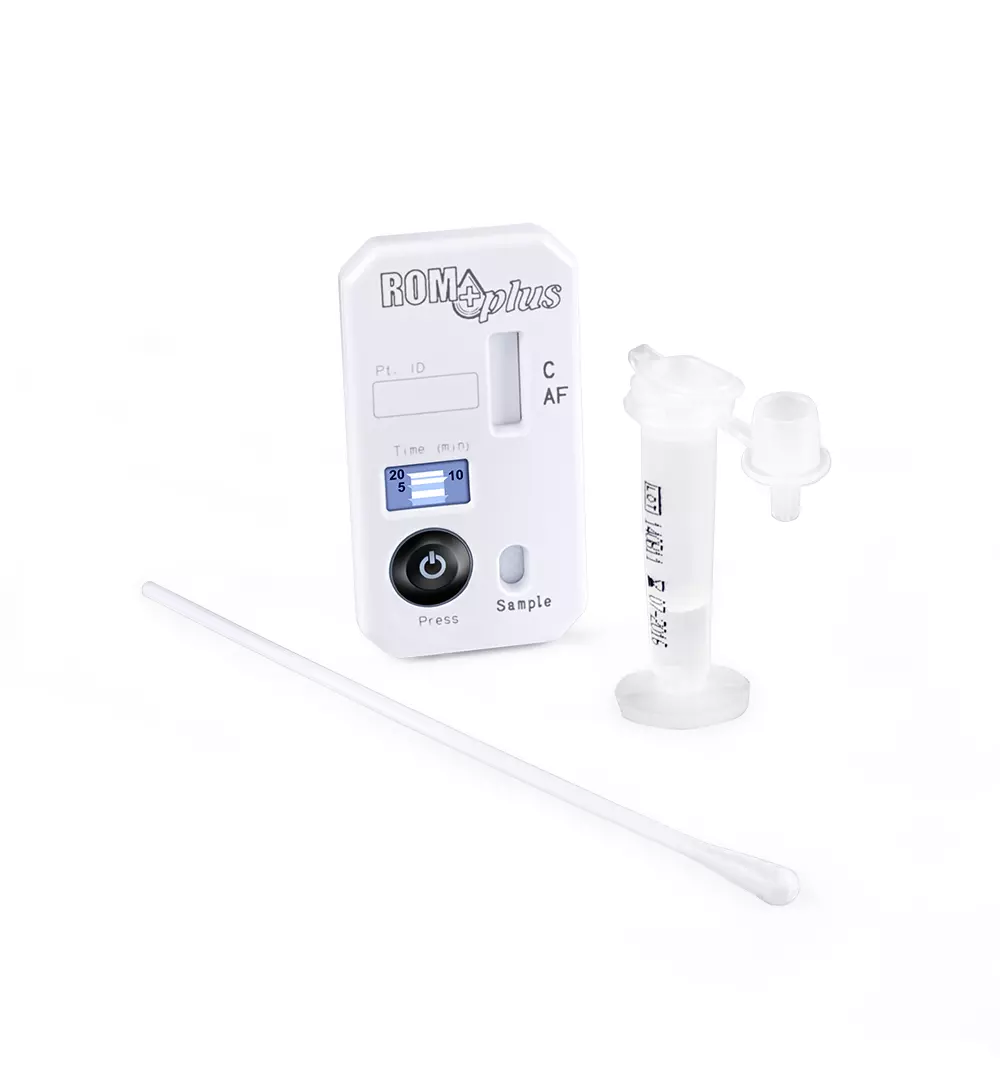
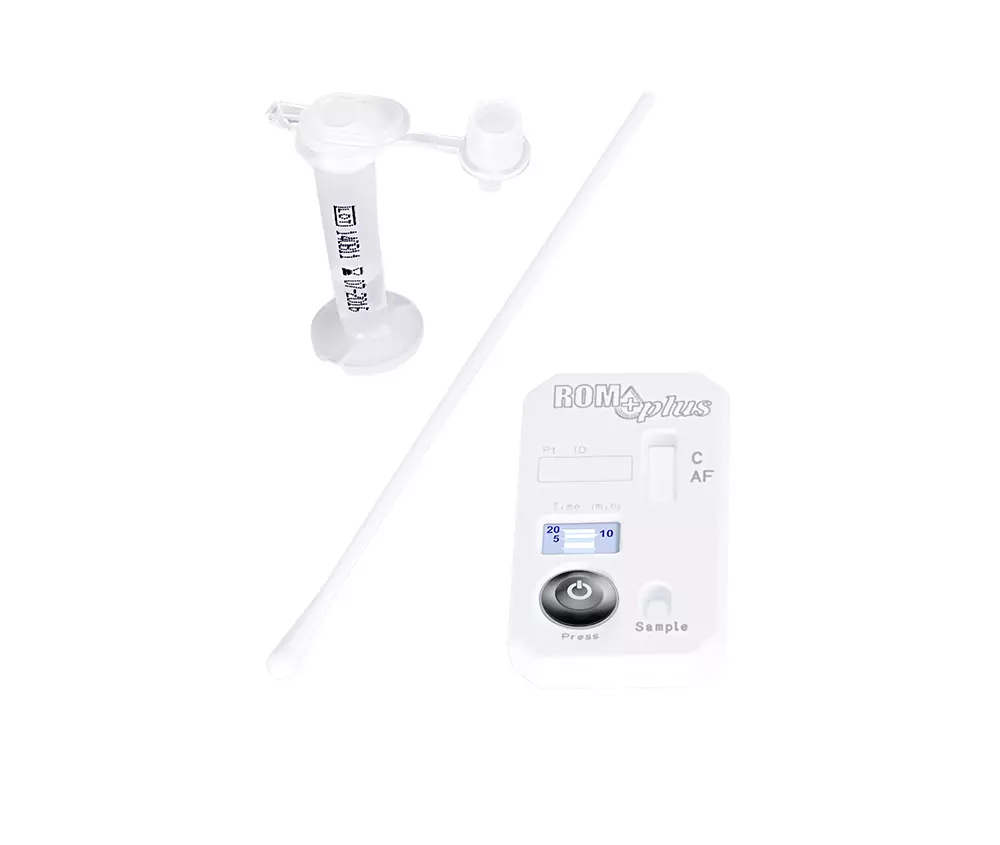
The ROM CS is a commercially available quality control material with known values,
When implementing a new system, such as ROM Plus, the facility must verify the accuracy of the new system. This is best done by comparison to truth, but if truth is not available, the ROM-CS can be used.
From the CLIA Certification of Performance Specifications Brochure #2:
“The laboratory needs to compare the accuracy of the test results it obtains when using a test system with the manufacturer’s accuracy claims. This can be done by testing commercially available calibrators/calibration and quality control materials with known values, proficiency testing materials that have established values, and previously tested patient specimens with established values. It test results for these samples fall within the manufacturers stated acceptable limits, accuracy is verified.”
The ROM CS kit comes with:
- 20 blinded samples
- Sealed known results
- ROM CS correlation log
- Instructions for use
The ROM CS samples are either positive or negative. CLIA regulations only require a positive or a negative sample and do not require low/high positives.
*ROM CS is only available for the ROM Plus® Rupture of Membranes Test in the US.