Revolutionizing The Treatment Of BPH
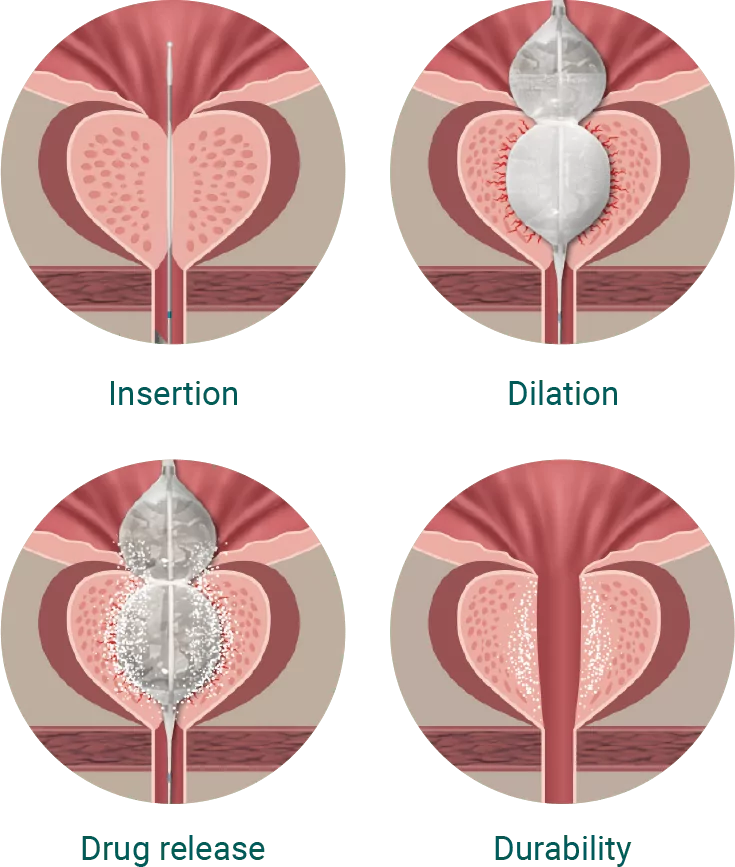
Optilume® BPH Catheter System is a minimally invasive procedure with quick recovery time, and immediate symptom relief that is durable. By combining mechanical dilation of the prostate with concurrent delivery of an anti-mitotic pharmaceutical agent, paclitaxel, your physician can effectively target and treat the cause of your BPH symptoms. After the Optilume ® BPH procedure, you will notice an immediate improvement in both urinary symptoms and quality of life, including improved urinary flow and the ability to happily relieve your bladder. Clinical studies show significant and durable improvements, allowing you to reclaim control of your urinary system.
Choose the Optilume® BPH Catheter System for immediate, lasting relief
- Clinical studies show Optilume® BPH is safe and effective (3,4)
- Immediate and durable symptom relief (3,4)
- Minimal catheter time (3,4)
- No impact on sexual function (3,4)
- Quick recovery (3,4)
- Highest clinically reported flow rates of any minimal invasive therapy (4)
- In-office/outpatient procedure
- No heating, burning, lasering, steaming, or implantation
After the Optilume® BPH procedure you will notice an immediate improvement in both urinary symptoms and quality of life, including improved urinary flow and the ability to happily relieve your bladder. Clinical studies show significant and durable improvements (3,4), allowing you to reclaim control of your urinary system.