THE LIFT YOU HAVE BEEN WAITING FOR!
Reliable Lift
- Margin delineation with darker color
- Cellulose-based and hypertonic
- Slower rate of absorption into the body due to water-retaining properties
Item Numbers:
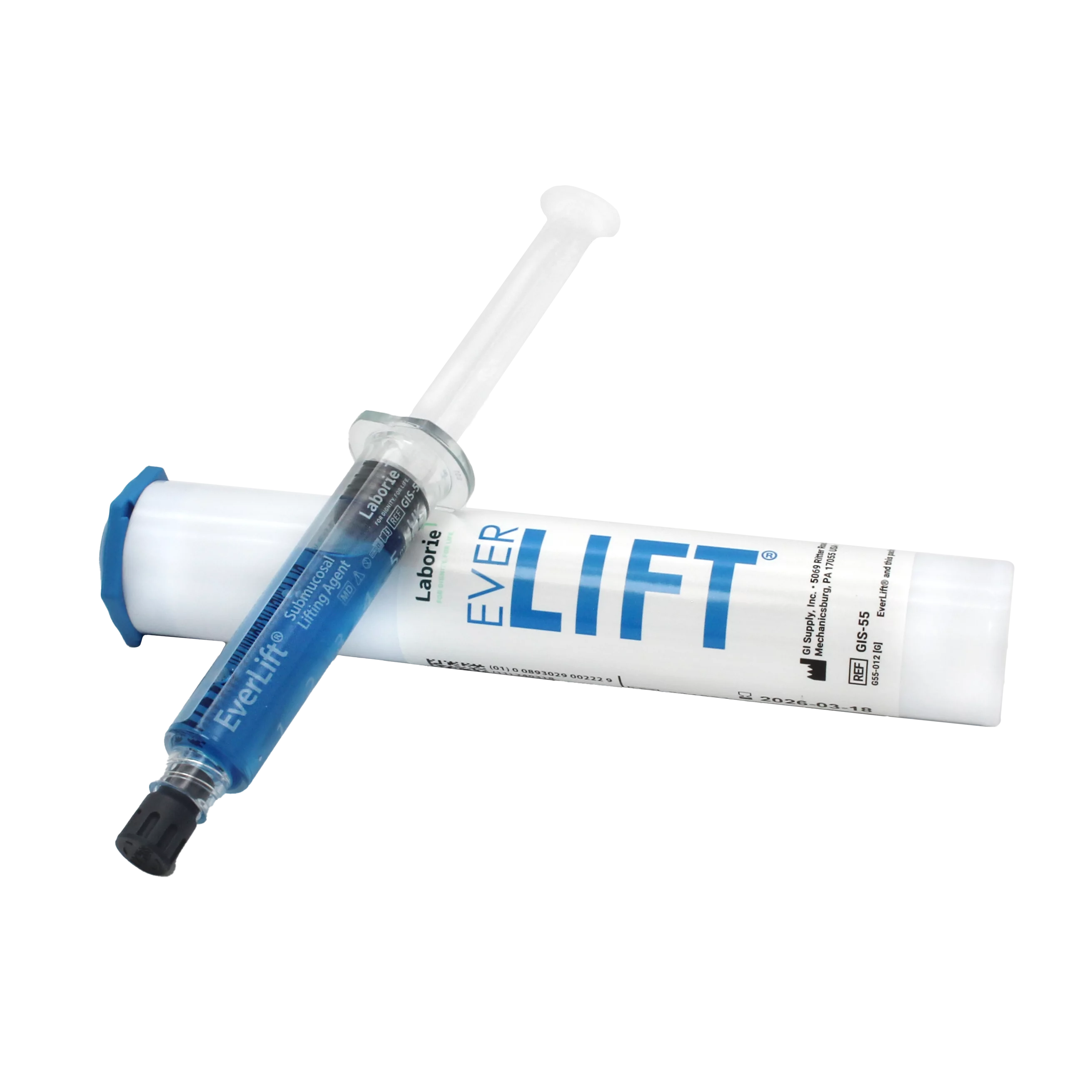
- GIS-55 (5 mL syringe)
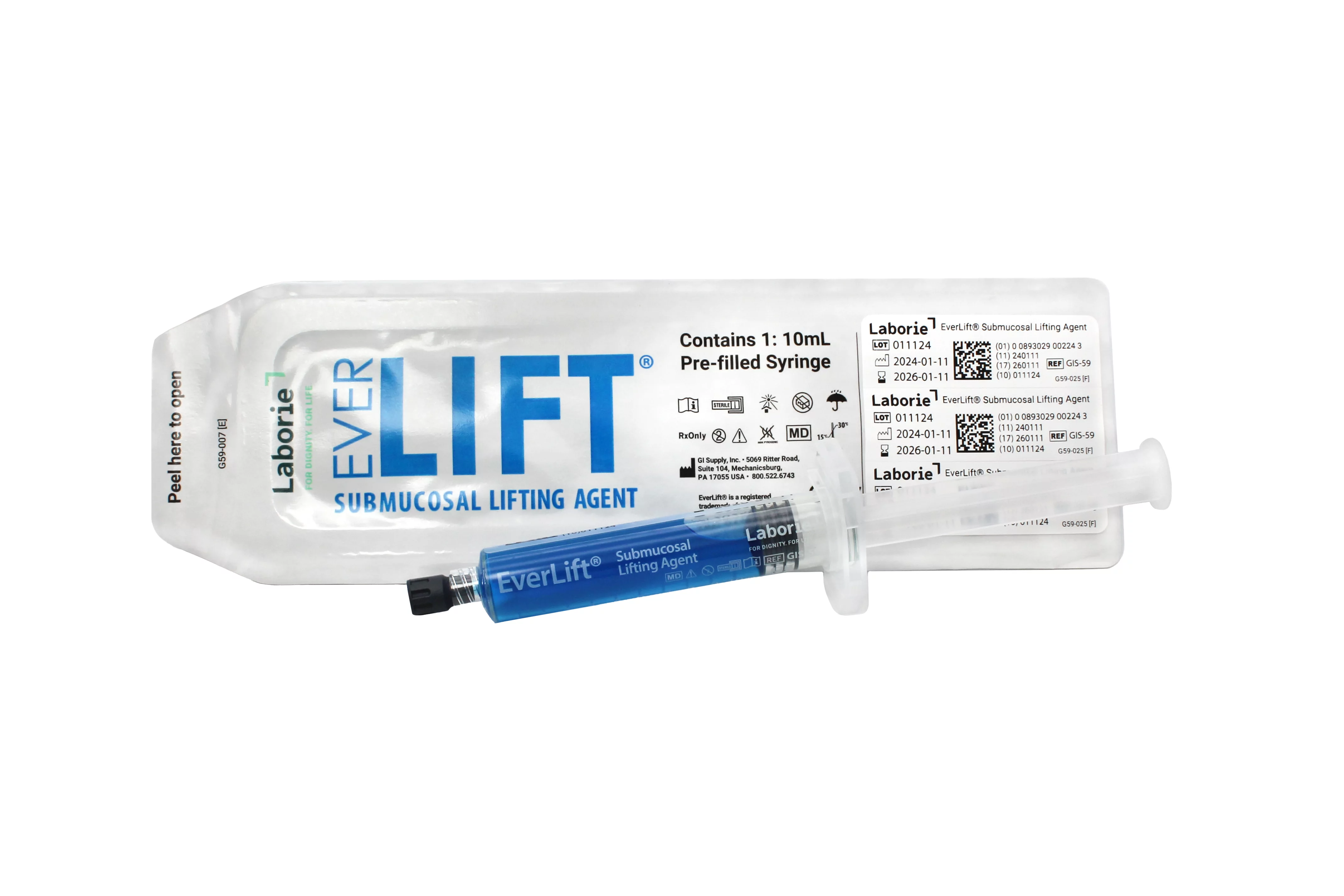
- GIS-59 (10 mL syringe)
Conveniently Packaged
- Pre-mixed with methylene blue
- Pre-filled and ready to use in 5 mL and 10 mL options
Cost Effective
- First submucosal lifting agent available in 5 mL and 10 mL sizes, allowing physicians to use only what they need, reducing costly waste
Product Details:
- Sterile, single patient use
- Made in the USA